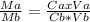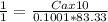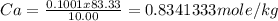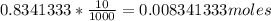Chemistry, 15.11.2019 19:31 jnsebastian2002
Vinegar is a dilute solution ethanoic acid in water (see the question above for the structure of ethanoic acid). in order to test the strength of a vinegar, a 10.00 gram sample was titrated with sodium hydroxide (0.1001 mole/kg of solution). the mass of the. burette before the titration is 131.44 g and upon reaching the endpoint the burette weighted 48.11 g. how many moles of acetic acid are in the sample?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, officialalex6330
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 14:30, isaiahrodriguezsm17
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

You know the right answer?
Vinegar is a dilute solution ethanoic acid in water (see the question above for the structure of eth...
Questions in other subjects:

Mathematics, 19.08.2019 22:40


Chemistry, 19.08.2019 22:40

French, 19.08.2019 22:40



English, 19.08.2019 22:40

History, 19.08.2019 22:40

Mathematics, 19.08.2019 22:40

Mathematics, 19.08.2019 22:40