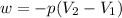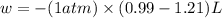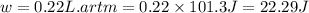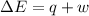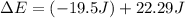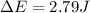Chemistry, 15.11.2019 17:31 lovemusic4
Ahelium-filled balloon at 310.0 k and 1 atm, contains 0.05 g he, and has a volume of 1.21 l. it is placed in a freezer (t = 235.0 k), and its volume decreases to 0.99 l. find δe for the gas (in joules). (cp of he = 20.8 j/mol k.)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1
You know the right answer?
Ahelium-filled balloon at 310.0 k and 1 atm, contains 0.05 g he, and has a volume of 1.21 l. it is p...
Questions in other subjects:










Mathematics, 07.05.2020 04:02

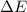 of the gas is 2.79 Joules.
of the gas is 2.79 Joules.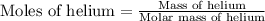
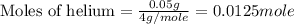
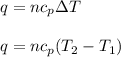
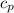 = specific heat of helium = 20.8 J/mol.K
= specific heat of helium = 20.8 J/mol.K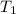 = initial temperature = 310.0 K
= initial temperature = 310.0 K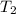 = final temperature = 235.0 K
= final temperature = 235.0 K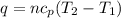
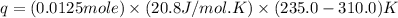
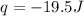
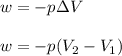
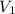 = initial volume = 1.21 L
= initial volume = 1.21 L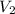 = final volume = 0.99 L
= final volume = 0.99 L