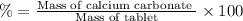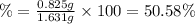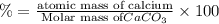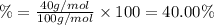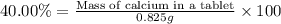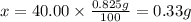Chemistry, 15.11.2019 07:31 harmonyfern5648
(a tablet containing calcium carbonate and fillers with a mass of 1.631 g was dissolved in hcl. after the fillers were filtered out, the hcl was neutralized by adding sodium carbonate. the resulting precipitate was pure calcium carbnonate (with the fillers removed). the solid calcium carbonate was collected on a watch glass that had a mass of 46.719 g when empty. after teh calcium carbonate had been allowed to dry, the mass of the watch glass plus product was found to be 47.544 g.
1. what is the mass of pure calcium carbonate product collected at the end of the experiment?
2. calculate the mass % calium carbonate in the tablet.
3. calculate the mass percent calcium in calcium carbonate. this calculation is theoretical and is independent of the data provided.
4. calculate the number of grams of calcium that were in the tablet.
(hint: this can be obtained by using the answers to question 1 and 3)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2
You know the right answer?
(a tablet containing calcium carbonate and fillers with a mass of 1.631 g was dissolved in hcl. afte...
Questions in other subjects:

English, 25.09.2020 03:01





Mathematics, 25.09.2020 03:01

Social Studies, 25.09.2020 03:01


Mathematics, 25.09.2020 03:01