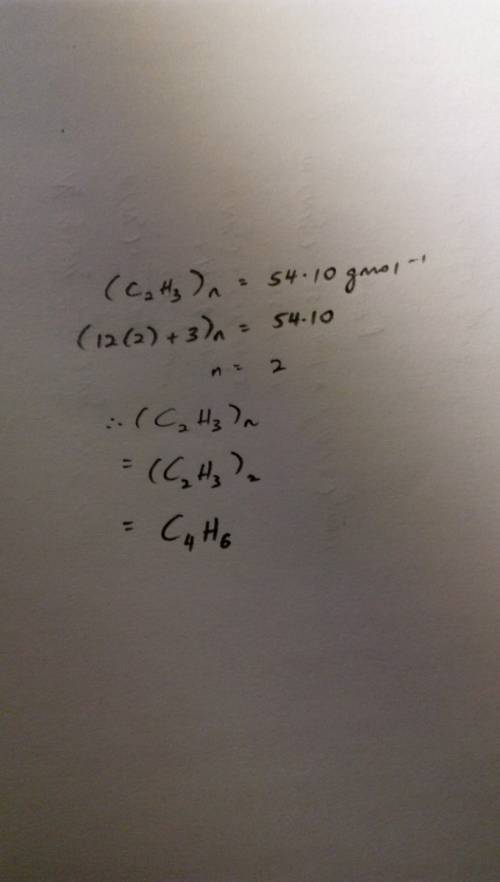Chemistry, 26.08.2019 12:30 kiarabermudez754
The empirical formula of a compound is determined to be c2h3, and its molecular mass is found to be 54.10 g/mol. determine the molecular formula of the compound, showing your work.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2
You know the right answer?
The empirical formula of a compound is determined to be c2h3, and its molecular mass is found to be...
Questions in other subjects:

Mathematics, 12.11.2020 22:30

Arts, 12.11.2020 22:30

Mathematics, 12.11.2020 22:30


Mathematics, 12.11.2020 22:30

Biology, 12.11.2020 22:30

History, 12.11.2020 22:30


Biology, 12.11.2020 22:30

Geography, 12.11.2020 22:30