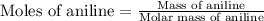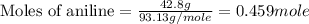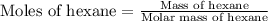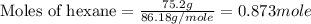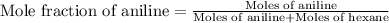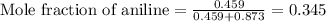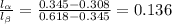Aniline and hexane form partially miscible liquid-liquid mixtures below 69 ºc. when 42.8 g of aniline and 75.2 g of hexane are mixed at 67.5 ºc, two separate liquid phases are formed, with mole fractions of aniline of 0.308 and 0.618. determine the overall mole fraction of aniline in the mixture, and then use the lever rule to determine the relative amounts of the two phases.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
Aniline and hexane form partially miscible liquid-liquid mixtures below 69 ºc. when 42.8 g of anilin...
Questions in other subjects:

Mathematics, 19.10.2019 12:10

Computers and Technology, 19.10.2019 12:10



Mathematics, 19.10.2019 12:10

Business, 19.10.2019 12:10