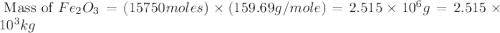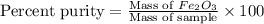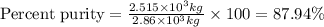One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is fe2o3 + 3co → 2fe + 3co2 suppose that 1.79 × 103 kg of fe is obtained from a 2.86 × 103 kg sample of fe2o3. assuming that the reaction goes to completion, what is the percent purity of fe2o3 in the original sample?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is...
Questions in other subjects:


Mathematics, 07.12.2021 02:40


Mathematics, 07.12.2021 02:40

Mathematics, 07.12.2021 02:40

English, 07.12.2021 02:40

Mathematics, 07.12.2021 02:40


History, 07.12.2021 02:40

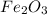 in the original sample is 87.94 %
in the original sample is 87.94 %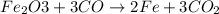
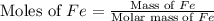
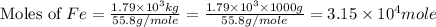
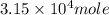 of Fe produced from
of Fe produced from  mole of
mole of