
Suppose that some fescn2+ is added to the above solution to shift the equilibrium. when equilibrium is re-established, the following concentrations are found.
a) [fe3+ ] = 8.12 ✕ 10−3 m,
b) [scn − ] = 7.84 ✕ 10−3 m
what is the concentration of fescn2+ in the new equilibrium mixture?
hint: use your value of k to solve for the unknown concentration.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2


Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3
You know the right answer?
Suppose that some fescn2+ is added to the above solution to shift the equilibrium. when equilibrium...
Questions in other subjects:

Mathematics, 15.12.2020 01:50

English, 15.12.2020 01:50


Chemistry, 15.12.2020 01:50

Mathematics, 15.12.2020 01:50

English, 15.12.2020 01:50



Mathematics, 15.12.2020 01:50

Mathematics, 15.12.2020 01:50

![[FeSCN^{2+}] \rightleftharpoons [Fe^{3+}] + [SCN^{-}]](/tpl/images/0374/6366/acc98.png)
![[Fe^{3+}] = 8.17 \times 10^{-3}](/tpl/images/0374/6366/d6b48.png) M
M![[SCN^{-}] = 8.60 \times 10^{-3}](/tpl/images/0374/6366/bf96b.png) M
M![[FeSCN^{2+}] = 6.25 \times 10^{-2}](/tpl/images/0374/6366/063d9.png) M
M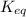 as follows.
as follows.![K_{eq} = \frac{[Fe^{3+}][SCN^{-}]}{[FeSCN^{2+}]}](/tpl/images/0374/6366/0a251.png)
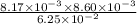

![[FeSCN^{2+}]](/tpl/images/0374/6366/797d4.png) will be calculated as follows.
will be calculated as follows.![11.24 \times 10^{-4} = \frac{8.12 \times 10^{-3} \times 7.84 \times 10^{-3}}{[FeSCN^{2+}]}](/tpl/images/0374/6366/07a9a.png)
![[FeSCN^{2+}] = 5.66 \times 10^{-2}](/tpl/images/0374/6366/abbb1.png) M
M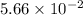 M.
M.

