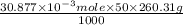Chemistry, 14.11.2019 20:31 SucMaDongShan
Using the henderson-hasselbalch equation, calculate the amount of hepes (sodium salt) and hepes (free acid) required to prepare 50 ml of a 100 mm buffer that is ph = 7.20. the pka of hepes is 7.55 at 20° c. the formula weight of the sodium salt is 260.31. the formula weight of the free acid is 238.31. weigh out the appropriate amounts of the hepes (sodium salt) and hepes (free acid), transfer to a 100 ml beaker, dissolve in deionized water to an approximate volume of 40 ml

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
Using the henderson-hasselbalch equation, calculate the amount of hepes (sodium salt) and hepes (fre...
Questions in other subjects:

English, 09.11.2020 14:00

Physics, 09.11.2020 14:00

History, 09.11.2020 14:00

Computers and Technology, 09.11.2020 14:00



History, 09.11.2020 14:00

English, 09.11.2020 14:00

English, 09.11.2020 14:00

Social Studies, 09.11.2020 14:00

![pK_{a} + log \frac{[Salt]}{[Acid]}](/tpl/images/0374/5521/81f72.png)
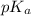 = 7.55.
= 7.55.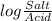
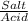 = 0.4467
= 0.4467 = 100 mM
= 100 mM 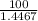
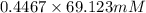
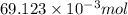 .
.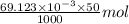
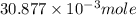 .
.