
Chemistry, 23.08.2019 20:40 hbkakabryce0p3fkoq
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each reaction :
a) 2na(s) + br2(l) > 2nabr(s)
b) h2(g) + cl2(g) > 2hcl(g)
c) 2li(s) + f2(g) > 2lif(s)
d) s(s) + cl2(g) > scl2(g)
e)n2(g) + 2o2(g) > 2no2(g)
f) mg(s) +cu(no3)2(aq) = mg(no3)2(aq) + cu(s)
for each reaction above, identify the reducing agent and the oxidizing agent

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, IsabellaGracie
True or false, the three major scales used to measure earthquakes are mercalli scale, richter scale and magnitude scale
Answers: 2


Chemistry, 22.06.2019 07:00, misspicafunpoke
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each rea...
Questions in other subjects:




Mathematics, 28.01.2020 19:54

Physics, 28.01.2020 19:54

History, 28.01.2020 19:54


Mathematics, 28.01.2020 19:54


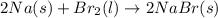
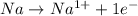
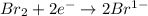
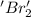 is reduced in this reaction. The reducing agent is, 'Na' and oxidizing agent is,
is reduced in this reaction. The reducing agent is, 'Na' and oxidizing agent is, 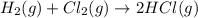
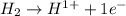

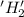 is oxidized and
is oxidized and 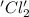 is reduced in this reaction. The reducing agent is,
is reduced in this reaction. The reducing agent is, 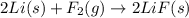
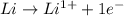
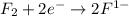
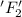 is reduced in this reaction. The reducing agent is, 'Li' and oxidizing agent is,
is reduced in this reaction. The reducing agent is, 'Li' and oxidizing agent is, 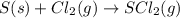
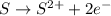
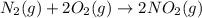
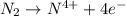

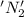 is oxidized and
is oxidized and 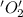 is reduced in this reaction. The reducing agent is,
is reduced in this reaction. The reducing agent is, 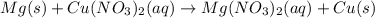
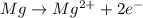
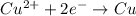
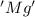 is oxidized and
is oxidized and 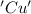 is reduced in this reaction. The reducing agent is, 'Mg' and oxidizing agent is, 'Cu'.
is reduced in this reaction. The reducing agent is, 'Mg' and oxidizing agent is, 'Cu'.

