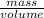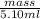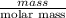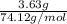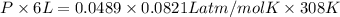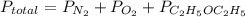Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l vessel that already contains a mixture of n2 and o2, whose partial pressures are pn2 = 0.752 atm and po2 = 0.206 atm. the temperature is held at 35.0 °c, and the diethylether totally evaporates.
a) calculate the partial pressure of the diethylether.
b) calculate the total pressure in the container.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 21.06.2019 20:50, deanlmartin
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2
You know the right answer?
Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l...
Questions in other subjects:






Biology, 22.12.2021 04:00

Physics, 22.12.2021 04:00

Physics, 22.12.2021 04:00

Biology, 22.12.2021 04:00

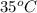 = (35 + 273) K = 308 K
= (35 + 273) K = 308 K