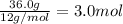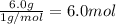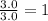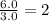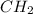Chemistry, 14.11.2019 17:31 KindaSmartPersonn
A42.0g sample of a compound containing only c and h was analyzed. the results showed that the sample contained 36.0g of c and 6.0g of h. which of the following questions about the compound can be answered using the results of the analysis?
a) what was the volume of the sample?
b) what is the molar mass of the compound?
c) what is the chemical stability of the compound?
d) what is the empirical formula of the compound?

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 23:30, billybob8514
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1
You know the right answer?
A42.0g sample of a compound containing only c and h was analyzed. the results showed that the sample...
Questions in other subjects:


Computers and Technology, 28.08.2019 16:00

Mathematics, 28.08.2019 16:00

Mathematics, 28.08.2019 16:00



Health, 28.08.2019 16:00

History, 28.08.2019 16:00