
Chemistry, 14.11.2019 06:31 strawberrymochi390
The reaction 2no2 → 2no + o2 obeys the rate law: rate = 1.4 x 10-2[no2]2 at 500 k . what would be the rate constant at 119 k if the activation energy is 80. kj/mol? this is a second order reaction, giving k the units of m-1s-1 this will not change with the change in temperature. do not include units in your answer. exponential numbers need to be entered like this: 2 e-1 means 2 x 10-1. the rate constant, k, at 119 k equals:

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, skinniestoflegends
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2


Chemistry, 23.06.2019 08:30, aambitiouss
Of element x has 22 protons, how many electrons does it have
Answers: 1
You know the right answer?
The reaction 2no2 → 2no + o2 obeys the rate law: rate = 1.4 x 10-2[no2]2 at 500 k . what would be t...
Questions in other subjects:


Computers and Technology, 09.03.2021 06:30

Mathematics, 09.03.2021 06:40

History, 09.03.2021 06:40


Mathematics, 09.03.2021 06:40



Mathematics, 09.03.2021 06:40

![Rate=1.4\times 10^{-2}[NO_2]^2](/tpl/images/0373/7605/5818c.png) ..........(1)
..........(1)![Rate=k[NO_2]^2](/tpl/images/0373/7605/d48ef.png) ............(2)
............(2)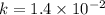
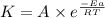
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0373/7605/6d953.png)
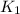 = rate constant at
= rate constant at 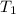 =
= 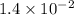
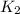 = rate constant at
= rate constant at 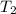 = ?
= ?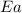 = activation energy for the reaction = 80.0 kJ/mole = 80000 J/mole
= activation energy for the reaction = 80.0 kJ/mole = 80000 J/mole![\log (\frac{K_2}{1.4\times 10^{-2}})=\frac{80000J/mole}{2.303\times 8.314J/mole.K}[\frac{1}{500}-\frac{1}{119}]](/tpl/images/0373/7605/44fe7.png)



