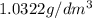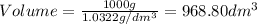1. for dry air at 1. atm pressure, the densities at –50°c, 0°c, and 69°c are 1.5826 g dm–3 , 1.2929 g dm–3, and 1.0322 g dm–3, respectively. a) assume a sample of mass 1000 g, and calculate the volume at each temperature. b) from these data, and assuming that air obeys charles’s law, determine a value for th

Answers: 2


Other questions on the subject: Chemistry



You know the right answer?
1. for dry air at 1. atm pressure, the densities at –50°c, 0°c, and 69°c are 1.5826 g dm–3 , 1.2929...
Questions in other subjects:

Mathematics, 08.04.2020 15:30




Health, 08.04.2020 15:30

Mathematics, 08.04.2020 15:30




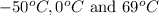 are
are 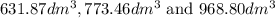 respectively.
respectively.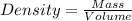
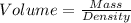
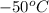 :
: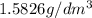
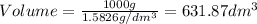
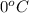 :
: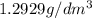
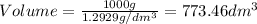
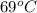 :
: