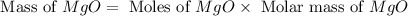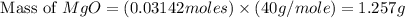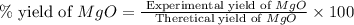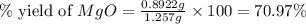Chemistry, 14.11.2019 06:31 yukichaniscool8
1. suppose 0.7542 g of magnesium reacts with excess oxygen to form magnesium oxide as the only product, what would be the theoretical yield of the product?
2. if 0.8922 g of magnesium oxide is obtained from the reaction indicated in #1 above, what would be the percent yield of the magnesium oxide?
3. suppose the percent yield was calculated to be over 100%, what possible reasons could you give to account for it?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
You know the right answer?
1. suppose 0.7542 g of magnesium reacts with excess oxygen to form magnesium oxide as the only produ...
Questions in other subjects:

Social Studies, 29.01.2020 13:02

Biology, 29.01.2020 13:02


Chemistry, 29.01.2020 13:02

Mathematics, 29.01.2020 13:02

Mathematics, 29.01.2020 13:02

Mathematics, 29.01.2020 13:02

Mathematics, 29.01.2020 13:02

History, 29.01.2020 13:02

History, 29.01.2020 13:02

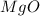 is, 1.257 grams.
is, 1.257 grams.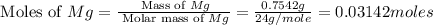
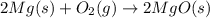
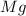 react to give 2 mole of
react to give 2 mole of