
Chemistry, 14.11.2019 04:31 jeanine239
Astudent who is performing this experiment pours an 8.50 ml sample of the saturated borax solution into a 10 ml graduated cylinder after the borax solution had cooled to a certain temperature t. the student rinses the sample into a small beaker using distilled water, and then titrates the solution with a 0.500 m hcl solution. 12.00 ml of the hcl solution is needed to reach the endpoint of the titration.
calculate the value of ksp for borax at temperature t.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, ayoismeisjjjjuan
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x. xx ml
Answers: 1

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3


You know the right answer?
Astudent who is performing this experiment pours an 8.50 ml sample of the saturated borax solution i...
Questions in other subjects:





Mathematics, 10.10.2021 17:30

Mathematics, 10.10.2021 17:30




Mathematics, 10.10.2021 17:30

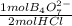 = 3,00x10⁻³ mol of B₄O₇²⁻
= 3,00x10⁻³ mol of B₄O₇²⁻

