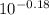Chemistry, 14.11.2019 03:31 THEOUTCOMER
Abuffer is prepared by adding 150 ml of 1.0 m naoh to 250 ml of 1.0 m nah2po4. how many moles of hcl must be added to this buffer solution to change the ph by 0.18 units? assume the total volume remains unchanged at 400 ml. for h2po4-, ka2 = 6.3 × 10^-8.
o 0.063 mol
o 1.0 mol
o 0.025 mol
o 0.082 mol
o 0.50 mol

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
Abuffer is prepared by adding 150 ml of 1.0 m naoh to 250 ml of 1.0 m nah2po4. how many moles of hcl...
Questions in other subjects:


Mathematics, 25.01.2021 06:10

English, 25.01.2021 06:10



Mathematics, 25.01.2021 06:10




Health, 25.01.2021 06:10