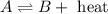H2 and i2 react in an exothermic reaction according to the following equation:
h2(g) + i2(g) double arrow yields 2hi (g)
h2 and i2 are placed in a sealed container and are allowed to reach equilibrium. what could you change about the system that would shift its equilibrium position?
a. add a catalyst.
b. decrease the volume.
c. increase the pressure.
d. lower the temperature.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 23.06.2019 02:30, erikacastro259
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 05:00, pmbeachy3102
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
You know the right answer?
H2 and i2 react in an exothermic reaction according to the following equation:
h2(g) + i2(g)...
h2(g) + i2(g)...
Questions in other subjects:




History, 03.03.2021 21:30



Advanced Placement (AP), 03.03.2021 21:30


History, 03.03.2021 21:30