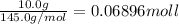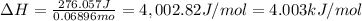Chemistry, 13.11.2019 19:31 danbat3023
Acoffee cup calorimeter was used to measure the heat of solution, the change in enthalpy that occurs when a solid dissolves in water. a 10.0 g sample of an ionic compound with a molar mass of 145.0 g/mol was added to a sample of deionized water to produce 60.0 grams of solution. after stirring and dissolving the solid, the temperature was found to change from 25.00 ∘c to 23.89 ∘c . calculate the enthalpy of solution, δhsoln , per mole of salt dissolved. assume the specific heat of the solution is 4.06 j/(g⋅∘c ) and the heat capacity of the calorimeter is 5.10 j/ ∘c . calculate the heat change experienced by the calorimeter contents, . = j calculate the heat change experienced by the calorimeter, . = j calculate the heat change produced by the solution process, . = j calculate δhsoln , the enthalpy of solution for one mole of solid in kilojoules per mole. δhsoln= kj/mol

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, microwave13016
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
Acoffee cup calorimeter was used to measure the heat of solution, the change in enthalpy that occurs...
Questions in other subjects:

Mathematics, 30.10.2020 01:30






Computers and Technology, 30.10.2020 01:30

Physics, 30.10.2020 01:30


Health, 30.10.2020 01:30

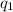
 = change in temperature = -1.11°C
= change in temperature = -1.11°C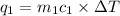
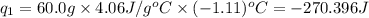
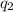
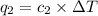

![Q=[-q_1+(-q_2)]](/tpl/images/0372/7156/e1e62.png)
![Q=[-(m_1c_1\times \Delta T)+(-c_2\times \Delta T)]](/tpl/images/0372/7156/ff7dd.png)
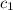 = specific heat of solution =
= specific heat of solution = 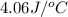
 = specific heat of calorimeter=
= specific heat of calorimeter= 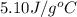
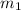 = mass of solution= 60.0 g
= mass of solution= 60.0 g![Q=[-(60.0 g\times 4.06J/g^oC\times (-1.11)^oC)+(- 5.10J/^oC\times (-1.11)^oC)]](/tpl/images/0372/7156/5fbbc.png)
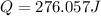
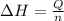
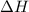 = enthalpy change = ?
= enthalpy change = ?