We expect the enthalpy of cumbustion of two isomers to be the molecular formulas of two molecules are so the balanced chemical equation for the two combustion reactions in calculation of combustion enthalpy from of products and reactants, the difference will be in the the
different, isomers, standard enthalpies of formation, combustion products, standard enthalpies of combustion, the same, very similar
the rod-shaped n-pentane has than the neopentane
tetrahedral, less, vibrational and rotational motions, more, translational motion, spherical

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, arodavoarodavo
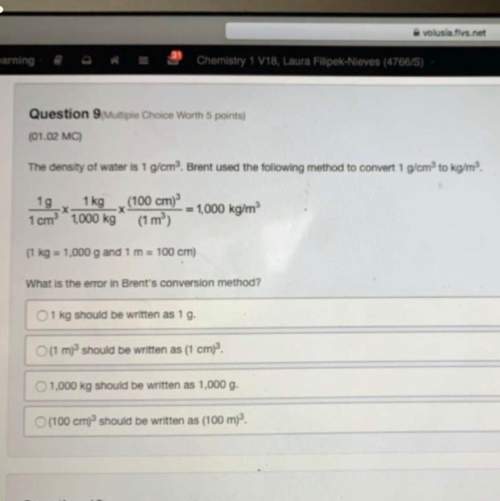
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3


Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
We expect the enthalpy of cumbustion of two isomers to be the molecular formulas of two molecules a...
Questions in other subjects:

Mathematics, 13.02.2021 01:40

Computers and Technology, 13.02.2021 01:40



History, 13.02.2021 01:40



Mathematics, 13.02.2021 01:40


Mathematics, 13.02.2021 01:40