
Chemistry, 13.11.2019 06:31 PrincessKehlani15
The oxidation of glucose can result in several products. you perform combustion analysis on a 2.750 g sample that resulted from the oxidation of glucose and obtain 3.702 g co2 and 1.514 g h2o. how many carbon atoms are in the empirical formula of the isolated sample? (assume no other products present from the oxidation of glucose.)]

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1

Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
The oxidation of glucose can result in several products. you perform combustion analysis on a 2.750...
Questions in other subjects:



Mathematics, 19.01.2021 22:00






Mathematics, 19.01.2021 22:00

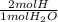 = 0.1682 mol H
= 0.1682 mol H

