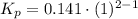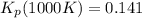Chemistry, 13.11.2019 06:31 SisterMina
Consider the equilibrium c2h6(g) ↔ c2h4(g) + h2(g) . at 1000k and a constant total pressure of 1 bar, h2(g) is introduced into the reaction vessel. the total pressure is held constant at 1 bar and at equilibrium the composition of the mixture in mole percent is h2 : 26% ; c2h4: 26% ; c2h6 : 48%calclate kp at 1000 k.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, smartie80
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3


Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
Consider the equilibrium c2h6(g) ↔ c2h4(g) + h2(g) . at 1000k and a constant total pressure of 1 bar...
Questions in other subjects:


Social Studies, 13.12.2021 17:50


Social Studies, 13.12.2021 17:50



Mathematics, 13.12.2021 17:50

Biology, 13.12.2021 17:50

Social Studies, 13.12.2021 17:50

SAT, 13.12.2021 17:50

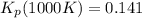
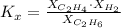
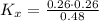
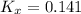
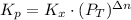
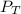 : total pressure and Δn: number of gaseous moles of product - number of gaseous moles of reactant
: total pressure and Δn: number of gaseous moles of product - number of gaseous moles of reactant