
Chemistry, 13.11.2019 04:31 friendsalwaysbae
The balanced chemical equation for the combustion of hexane is: 2c6h14(l) 19o2(g) 12co2(g) 14h2o(g) hexane is a low-boiling point component of gasoline. answer the question in part 1 through part 3, if the rate of reaction of c6h14 is 1.95 mol l-1s-1. the rate of reaction of c6h14 is the rate of disappearance of c6h14. what was the rate of formation of co2?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 07:30, Ayyyyeeeeeeewuzgud
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1
You know the right answer?
The balanced chemical equation for the combustion of hexane is: 2c6h14(l) 19o2(g) 12co2(g) 14h2o(g)...
Questions in other subjects:

History, 20.12.2020 04:10


Business, 20.12.2020 04:10



Mathematics, 20.12.2020 04:10

French, 20.12.2020 04:10

Mathematics, 20.12.2020 04:10


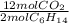 = 11,7 M/s
= 11,7 M/s

