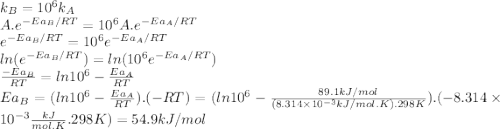Chemistry, 13.11.2019 02:31 joseperez1224
The standard free energy of activation of a reaction a is 81.9 kj mol–1 (19.6 kcal mol–1) at 298 k. reaction b is one million times faster than reaction a at the same temperature. the products of each reaction are 10.0 kj mol–1 (2.39 kcal mol–1) more stable than the reactants. (a) what is the standard free energy of activation of reaction b?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, llamasking
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

You know the right answer?
The standard free energy of activation of a reaction a is 81.9 kj mol–1 (19.6 kcal mol–1) at 298 k....
Questions in other subjects:


Mathematics, 04.02.2021 22:30

History, 04.02.2021 22:30


Mathematics, 04.02.2021 22:30



English, 04.02.2021 22:30

Chemistry, 04.02.2021 22:30

Mathematics, 04.02.2021 22:30


 .
.