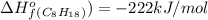Chemistry, 13.11.2019 00:31 ridzrana02
For a particular isomer of c8h18,c8h18, the combustion reaction produces 5099.5 kj 5099.5 kj of heat per mole of c8h18(g)c8h18(g) consumed, under standard conditions. c8h18(g)+252o2(g)⟶8co2(g)+9h2o(g)δh ∘rxn=−5099.5 kj/mol c8h18(g)+252o2(g)⟶8co2(g)+9h2o(g)δh rxn°=−5099.5 kj/mol what is the standard enthalpy of formation of this isomer of c8h18(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
For a particular isomer of c8h18,c8h18, the combustion reaction produces 5099.5 kj 5099.5 kj of heat...
Questions in other subjects:




Mathematics, 16.02.2021 18:50

Mathematics, 16.02.2021 18:50

History, 16.02.2021 18:50




Mathematics, 16.02.2021 18:50

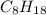 is -222 kJ/mol
is -222 kJ/mol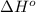
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0371/3800/45485.png)
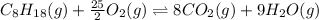
![\Delta H^o_{rxn}=[(n_{(CO_2)}\times \Delta H^o_f_{(CO_2)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})]-[(n_{(C_8H_{18})}\times \Delta H^o_f_{(C_8H_{18})})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0371/3800/70c63.png)
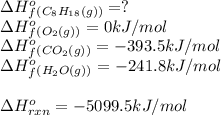
![-5099.5=[(8\times -393.5)+(9\times -241.5)]-[(1\times \Delta H^o_f_{(C_8H_{18})}))+(\frac{25}{2}\times 0)]](/tpl/images/0371/3800/1a9fc.png)