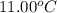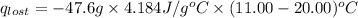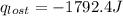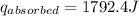A6.60 g sample of solid kcl was dissolved in 47.6 g of water. the initial temperature of the water was 20.00°c. after the compound dissolved, the temperature of the water was 11.00°c. assume the heat was completely absorbed from the water and no heat was absorbed by the reaction container or the surroundings. calculate the heat absorbed by the process. the specific heat of water is 4.184 j/g·°c.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 18:00, AdoNice
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
A6.60 g sample of solid kcl was dissolved in 47.6 g of water. the initial temperature of the water w...
Questions in other subjects:


Mathematics, 20.05.2020 15:58

Mathematics, 20.05.2020 15:58

Business, 20.05.2020 15:58



Mathematics, 20.05.2020 15:58


Mathematics, 20.05.2020 15:58

Mathematics, 20.05.2020 15:58

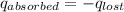
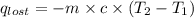
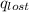 = heat lost by the water = ?
= heat lost by the water = ?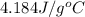
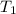 = initial temperature of water =
= initial temperature of water = 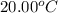
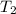 = final temperature of water =
= final temperature of water =