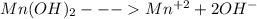Chemistry, 12.11.2019 23:31 chassidytjtrimb
Compare the solubility of mn(oh)2 (ksp = 1.6x10-13)in the following solutions: pure water, a solution buffered to be ph = 6.5 a solution buffered to be ph = 3.5 compare the solubility of mn(oh)2 (ksp = 1.6x10-13)in the following solutions: pure water, a solution buffered to be ph = 6.5 a solution buffered to be ph = 3.5 pure water > ph 3.5 > ph 6.5 (least soluble) ph 3.5 > ph 6.5 > pure water (least soluble) ph 3.5 > pure water > ph 6.5 (least soluble) pure water > ph 6.5 > ph 3.5 (least soluble) ph 6.5 > ph 3.5 > pure water (least soluble)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1

Chemistry, 22.06.2019 16:50, TrueKing184
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 23.06.2019 01:30, bgarrison364
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Compare the solubility of mn(oh)2 (ksp = 1.6x10-13)in the following solutions: pure water, a soluti...
Questions in other subjects:


Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10


Social Studies, 18.03.2021 01:10

Chemistry, 18.03.2021 01:10



Computers and Technology, 18.03.2021 01:10