
Chemistry, 12.11.2019 21:31 jeremyrs101
The standard state of phosphorus at 25∘c is p4. this molecule has four equivalent p atoms, no double or triple bonds, and no expanded octets. draw its lewis structure. draw the molecule by placing atoms on the grid and connecting them with bonds. include all non-bonding electrons.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1


You know the right answer?
The standard state of phosphorus at 25∘c is p4. this molecule has four equivalent p atoms, no double...
Questions in other subjects:

Mathematics, 14.07.2020 01:01




Mathematics, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01

Chemistry, 14.07.2020 01:01


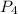 molecule.the
molecule.the  hybridized phosphorus atom lies in the corner of the regular tetrahedron. Each phosphorus atom is connected to three other phosphorus atoms.
hybridized phosphorus atom lies in the corner of the regular tetrahedron. Each phosphorus atom is connected to three other phosphorus atoms.


