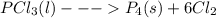Chemistry, 12.11.2019 06:31 gabrielolivas59
4pcl₃(l)  p₄(s) + 6cl₂(g); δh= 304.0 kj
p₄(s) + 6cl₂(g); δh= 304.0 kj
based on the reaction shown, which statement is true?
a) when 1 mol pcl₃(l) reacts, 304.0 kj are released. b) when 1 mol p₄(s) is produced, 304.0 kj are consumed. c) when 548.56 g pcl₃(l) react, 304.0 kj are released. d) when 137.14 g pcl₃(l) react, 304.0 kj are consumed. e) when 123.88 g p₄(s) are produced, 304.0 kj are released.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1
You know the right answer?
4pcl₃(l) [tex]\longrightarrow[/tex] p₄(s) + 6cl₂(g); δh= 304.0 kj
based on the reaction shown...
based on the reaction shown...
Questions in other subjects:



Mathematics, 25.02.2020 05:05

Mathematics, 25.02.2020 05:06

Mathematics, 25.02.2020 05:06



Mathematics, 25.02.2020 05:07


Mathematics, 25.02.2020 05:07