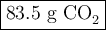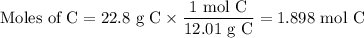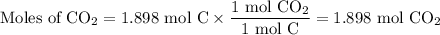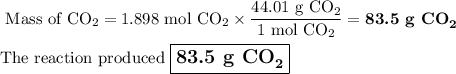Chemistry, 11.11.2019 23:31 jaylinthornton6
When 22.8 g of carbon were burned in the presence of 78. 9 g of oxygen 18.1 g of oxygen remained unreacted what mass of carbon dioxide was produced

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2



Chemistry, 23.06.2019 00:40, joe7977
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
When 22.8 g of carbon were burned in the presence of 78. 9 g of oxygen 18.1 g of oxygen remained unr...
Questions in other subjects:

Mathematics, 23.09.2019 07:30



Mathematics, 23.09.2019 07:30


Social Studies, 23.09.2019 07:30

Mathematics, 23.09.2019 07:30

English, 23.09.2019 07:30

Mathematics, 23.09.2019 07:30