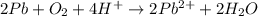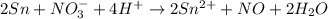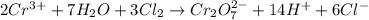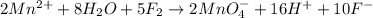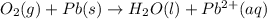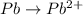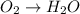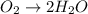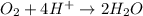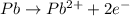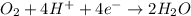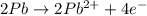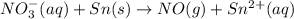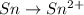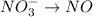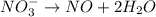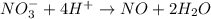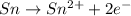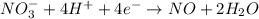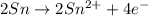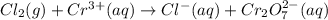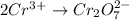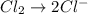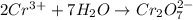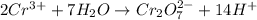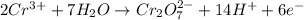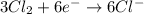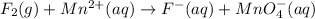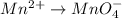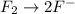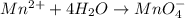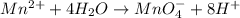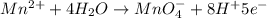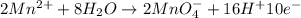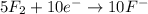Balance the following oxidation-reduction reactions, which occur in acidic solution, using the half-reaction method.
(use the lowest possible coefficients. include states-of-matter under the given conditions in your answer.)
o2(g) + pb(s) → h2o(l) + pb2+(aq) (b) no3−(aq) + sn(s) → no(g) + sn2+(aq) (c) cl2(g) + cr3+(aq) → cl −(aq) + cr2o72−(aq) (d) f2(g) + mn2+(aq) → f −(aq) + mno4−(aq)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 05:40, girlchamp654
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2

Chemistry, 23.06.2019 05:50, starfox5454
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1


Chemistry, 23.06.2019 15:00, Fangflora3
What do we call the rows on the periodic table? a. periodb. familyc. groupd. metals
Answers: 1
You know the right answer?
Balance the following oxidation-reduction reactions, which occur in acidic solution, using the half-...
Questions in other subjects:

Mathematics, 18.01.2020 02:31




Chemistry, 18.01.2020 02:31

Mathematics, 18.01.2020 02:31



Mathematics, 18.01.2020 02:31

History, 18.01.2020 02:31