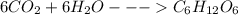The reaction for producing glucose in plants, called photosynthesis, is 6co2+6h2o−→−−lightc6h12o6+6o2 if a plant produces 4.36 mol c6h12o6, how many moles of h2o are needed? moles h2o : the reaction for producing glucose in plants, called photosynthesis, is 6co2+6h2o−→−−lightc6h12o6+6o2 if a plant produces 4.36 mol c6h12o6, how many moles of h2o are needed? moles h2o :

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, gatorr2010
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 03:30, babygirl1780
Nanotechnology, the field of trying to build ultrasmall structures one atom at a time, has progressed in recent years. one potential application of nanotechnology is the construction of artificial cells. the simplest cells would probably mimic red blood cells, the body's oxygen transporters. for example, nanocontainers, perhaps constructed of carbon, could be pumped full of oxygen and injected into a person's bloodstream. if the person needed additional oxygen-due to a heart attack perhaps, or for the purpose of space travel-these containers could slowly release oxygen into the blood, allowing tissues that would otherwise die to remain alive. suppose that the nanocontainers were cubic and had an edge length of 24 nanometers. part a part complete what is the volume of one nanocontainer? (ignore the thickness of the nanocontainer's wall.) express your answer using two significant figures. v v = 1.4ă—10â’20 l previous answers correct significant figures feedback: your answer 1.3824â‹…10â’20 = 1.382ă—10â’20 l was either rounded differently or used a different number of significant figures than required for this part. if you need this result for any later calculation in this item, keep all the digits and round as the final step before submitting your answer. part b suppose that each nanocontainer could contain pure oxygen pressurized to a density of 81 g/l . how many grams of oxygen could be contained by each nanocontainer?
Answers: 3

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 00:30, terryg4397
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
The reaction for producing glucose in plants, called photosynthesis, is 6co2+6h2o−→−−lightc6h12o6+6o...
Questions in other subjects:



Mathematics, 28.04.2021 14:00

English, 28.04.2021 14:00


Chemistry, 28.04.2021 14:00

Mathematics, 28.04.2021 14:00


Biology, 28.04.2021 14:00