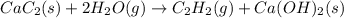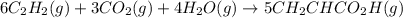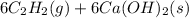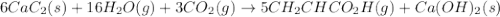There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide:
cac2(s)+2h2o(g)→c2h2(g)+caoh2(s)
in the second step, acetylene, carbon dioxide and water react to form acrylic acid:
6c2h2(g)+3co2(g)+4h2o(g)→5ch2chco2h (g)
write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. be sure your equation is balanced.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of...
Questions in other subjects:

Geography, 05.10.2019 02:30

Geography, 05.10.2019 02:30





Geography, 05.10.2019 02:30