Chemistry, 10.11.2019 06:31 Alienchild239
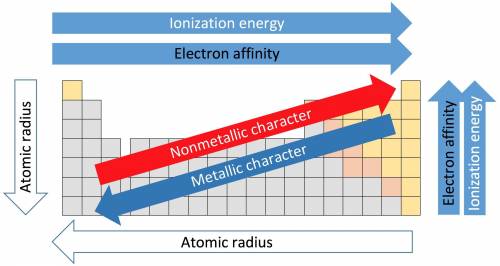
How do the periodic trends in metallic character compare to those for ionization energy? how do the periodic trends in metallic character compare to those for ionization energy? metals tend to have higher ionization energies than nonmetals. metals tend to have lower ionization energies than nonmetals. metals and nonmetals tend to have the same ionization energies.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, flowergirly34
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1


Chemistry, 23.06.2019 02:00, hermesrobles
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
You know the right answer?
How do the periodic trends in metallic character compare to those for ionization energy? how do the...
Questions in other subjects:



English, 21.02.2021 08:00

Mathematics, 21.02.2021 08:00

Mathematics, 21.02.2021 08:00

Mathematics, 21.02.2021 08:00



English, 21.02.2021 08:00