
Chemistry, 10.11.2019 05:31 jalenclarke25
The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2co3) and the bicarbonate (hydrogen carbonate, hco3-) ion. what is the ratio of [bicarbonate]/[carbonic acid] at this ph? for carbonic acid, ka1 = 4.2 10-7.a) [bicarbonae]/[carbonic acid] = 0.11.b) [bicarbonate]/[carbonic acid] = 9.4.c) [bicarbonate]/[carbonic acid] = 0.38.d) none of the above ratios is correct. e) [bicarbonate]/[carbonic acid] = 2.65.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 20:10, maddie1776
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1


Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2...
Questions in other subjects:


Mathematics, 19.06.2021 01:30

Biology, 19.06.2021 01:30





Chemistry, 19.06.2021 01:30


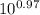 = [bicarbonate] / [carbonic acid] [bicarbonate] / [carbonic acid] = 9.33 ≈ 9.4
= [bicarbonate] / [carbonic acid] [bicarbonate] / [carbonic acid] = 9.33 ≈ 9.4

