
Chemistry, 10.11.2019 03:31 julliette27
An ice cube of mass 9.0 g at temperature 0∘c is added to a cup of coffee, whose temperature is 90 ∘c and which contains 110 g of liquid. assume the specific heat capacity of the coffee is the same as that of water. the heat of fusion of ice (the heat associated with ice melting) is 6.0 kj/mol.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1


Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2
You know the right answer?
An ice cube of mass 9.0 g at temperature 0∘c is added to a cup of coffee, whose temperature is 90 ∘c...
Questions in other subjects:

Mathematics, 16.04.2021 22:20


Mathematics, 16.04.2021 22:20




Chemistry, 16.04.2021 22:20

Mathematics, 16.04.2021 22:20


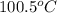

 = specific heat of coffee = specific heat of water =
= specific heat of coffee = specific heat of water =  (as per question)
(as per question) = specific heat of ice =
= specific heat of ice = 
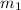 = mass of coffee = 110 g
= mass of coffee = 110 g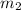 = mass of ice = 9.0 g
= mass of ice = 9.0 g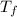 = final temperature = ?
= final temperature = ? = initial temperature of coffee =
= initial temperature of coffee = 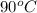
 = initial temperature of ice =
= initial temperature of ice = 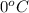
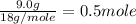
 = 6.0 kJ/mol = 6000 J/mol
= 6.0 kJ/mol = 6000 J/mol




