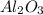Chemistry, 10.11.2019 03:31 babyboogrocks5572
For the following reaction, 4.07 grams of aluminum oxide are mixed with excess sulfuric acid. the reaction yields 10.4 grams of aluminum sulfate. aluminum oxide (s) + sulfuric acid (aq) aluminum sulfate (aq) + water (l) what is the theoretical yield of aluminum sulfate? grams what is the percent yield for this reaction? %

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
For the following reaction, 4.07 grams of aluminum oxide are mixed with excess sulfuric acid. the re...
Questions in other subjects:




Mathematics, 07.04.2021 19:50

Mathematics, 07.04.2021 19:50

Mathematics, 07.04.2021 19:50


English, 07.04.2021 19:50


English, 07.04.2021 19:50