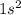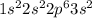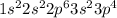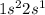Agouy balance is used to determine the magnetic susceptibility of a substance. a strong electromagnet is placed next to the sample, which is on a balance. if the sample is paramagnetic, the mass reading of the balance will increase when the field is switched on. based on their electron configurations, predict whether these elements are paramagnetic or diamagnetic.
paramagnetic
he ,mg ,s ,li

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 06:30, morganzahn16
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
You know the right answer?
Agouy balance is used to determine the magnetic susceptibility of a substance. a strong electromagne...
Questions in other subjects:


English, 03.05.2020 13:16


History, 03.05.2020 13:16


History, 03.05.2020 13:16



Mathematics, 03.05.2020 13:16

Mathematics, 03.05.2020 13:16