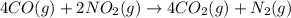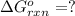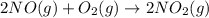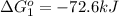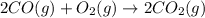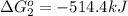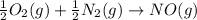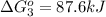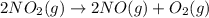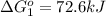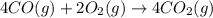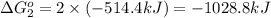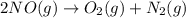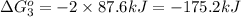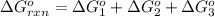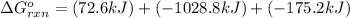Chemistry, 10.11.2019 01:31 TheOriginal2x
Calculate δg∘rxn for the following reaction: 4co(g)+2no2(g)→4co2(g)+n2(g).use the following reactions and given δg∘rxn values: a) 2no(g)+o2(g)→2no2(g), δg∘rxn= - 72.6 kjb) 2co(g)+o2(g)→2co2(g), δg∘rxn= - 514.4 kjc) 12o2(g)+12n2(g)→no(g), δg∘rxn= 87.6 kj

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Falconpride4079
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1

Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 02:10, apowers6361
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3
You know the right answer?
Calculate δg∘rxn for the following reaction: 4co(g)+2no2(g)→4co2(g)+n2(g).use the following reaction...
Questions in other subjects:


Physics, 02.11.2020 08:30




Biology, 02.11.2020 08:30


English, 02.11.2020 08:30

Biology, 02.11.2020 08:30

Mathematics, 02.11.2020 08:30

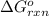 for the reaction is -1131.4 kJ
for the reaction is -1131.4 kJ