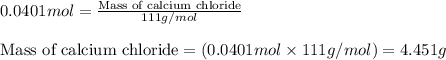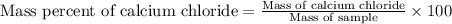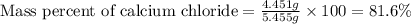Chemistry, 09.11.2019 08:31 Caixiayang3613
1. a 5.455-g sample of impure cacl2 is dissolved and treated with excess potassium carbonate solution. the dried caco3 (calcium carbonate) precipitate weighs 4.010-g. calculate the percent by mass of cacl2 in the original mixture.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, richardwalker8ourhg2
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a. the mitochondria b. the nucleus c. the vacuoles d. the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 05:30, khaylaperry
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
1. a 5.455-g sample of impure cacl2 is dissolved and treated with excess potassium carbonate solutio...
Questions in other subjects:


English, 05.10.2020 02:01



Health, 05.10.2020 02:01

Mathematics, 05.10.2020 02:01


History, 05.10.2020 02:01


Mathematics, 05.10.2020 02:01

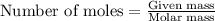 .....(1)
.....(1)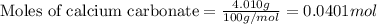
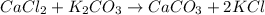
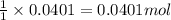 of calcium chloride
of calcium chloride