
Chemistry, 09.11.2019 05:31 elijahlylejamez45
Ammonia is produced commercially by the direct reaction of the elements. the formation of 1.00 moles of gaseous nh3 by this reaction releases 46.17 kj of heat. how much energy (in kj) is released, when 28.0 kg of hydrogen gas, h2, reacts in excess nitrogen gas, n2?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, ayoismeisalex
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 01:00, morrisjillian23
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 04:30, KarenH3512
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1
You know the right answer?
Ammonia is produced commercially by the direct reaction of the elements. the formation of 1.00 moles...
Questions in other subjects:

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

History, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 15:01

 of heat energy will be released
of heat energy will be released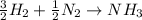
 moles of
moles of  produces 1 mol of
produces 1 mol of  to release 46.17 kJ of heat.
to release 46.17 kJ of heat. moles of
moles of  moles of
moles of  heat or
heat or 

