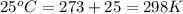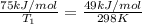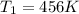Hydrogen peroxide decomposes spontaneously to yield water and oxygen gas according to the following reaction equation. 2h202(aq) →2h20(/) + 02(g) the activation energy for this reaction is 75 kj/mol. in the presence of a metal catalyst the activation energy is lowered to 49 kj/mol. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the metal-catalyzed reaction at 25°c?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, alevans7144
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 00:10, goodygoodgirlygirl
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 04:30, logan12345677885675
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2
You know the right answer?
Hydrogen peroxide decomposes spontaneously to yield water and oxygen gas according to the following...
Questions in other subjects:


Mathematics, 30.05.2020 06:00


Mathematics, 30.05.2020 06:00






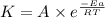
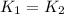

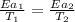 ..........(1)
..........(1)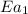 = activation energy for non-catalyzed reaction = 75 kJ/mol
= activation energy for non-catalyzed reaction = 75 kJ/mol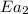 = activation energy for catalyzed reaction = 49 kJ/mol
= activation energy for catalyzed reaction = 49 kJ/mol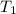 = temperature for non-catalyzed reaction = ?
= temperature for non-catalyzed reaction = ?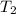 = temperature for catalyzed reaction =
= temperature for catalyzed reaction =