
Chemistry, 08.11.2019 23:31 astultz309459
Suppose 200.0 ml of 1.00 m hcl and 200.0 ml of 1.00 m naoh, both initially at 21.0°c, are mixed in a thermos flask. when the reaction is complete, the temperature is 27.8°c. assuming that the solutions have the same heat capacity as pure water, compute the heat released (in kj).

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3

Chemistry, 23.06.2019 14:00, RichardKing2376
Tinererining 01: 57: 44 which statement correcte describes the actual veld and the theoretical yield of a reaction? textual vec is calculated to the reactant amounts but the theoretical yeld must be measured for each instance of a the actual vec is calculated from the amount of the limiting reactant and the theoretical yield is calculated from the 发公主 the actual weld depends on the reaction centers, but the theoretical yield and only with reactant amounts the actual vele represents the maximum weld possible and the theoretical yield assumes perfect reaction conditions save and ext e அட
Answers: 2

Chemistry, 23.06.2019 20:30, chayaharroch03
Due tomorrow write the chemical equation that has the equilibrium constant expression [listed in photo]
Answers: 2
You know the right answer?
Suppose 200.0 ml of 1.00 m hcl and 200.0 ml of 1.00 m naoh, both initially at 21.0°c, are mixed in a...
Questions in other subjects:


Chemistry, 11.03.2021 19:30

Mathematics, 11.03.2021 19:30

Mathematics, 11.03.2021 19:30



Mathematics, 11.03.2021 19:30


Business, 11.03.2021 19:30


 = 200 g
= 200 g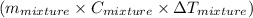
 represents change in temperature
represents change in temperature )
)![[400g\times 4.186J.g^{-1}.^{0}\textrm{C}^{-1}\times(27.8-21.0)^{0}\textrm{C} ]](/tpl/images/0366/2490/9c7ad.png) =
=  J=11.4 kJ
J=11.4 kJ

