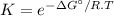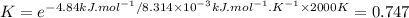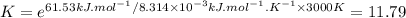Chemistry, 08.11.2019 05:31 ekerns2000paa19x
The decomposition of a generic diatomic element in its standard state is represented by the equation 12x2(g)⟶x(g) assume that the standard molar gibbs energy of formation of x(g) is 4.84 kj·mol−1 at 2000 . k and −61.53 kj·mol−1 at 3000 . k. determine the value of the thermodynamic equilibrium constant, k , at each temperature. at 2000 . k, δ=4.84 kj·mol−1 . what is k at that temperature?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 06:20, Lindsay882
Type the correct answer in each box. balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
The decomposition of a generic diatomic element in its standard state is represented by the equation...
Questions in other subjects:



Mathematics, 05.09.2020 22:01


History, 05.09.2020 22:01

History, 05.09.2020 22:01


Mathematics, 05.09.2020 22:01