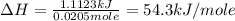Chemistry, 08.11.2019 04:31 AaronEarlMerringer
In the laboratory a general chemistry student finds that when 2.84 g of kclo4(s) are dissolved in 107.70 g of water, the temperature of the solution drops from 22.82 to 20.34 °c. the heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.55 j/°c. based on the student's observation, calculate the enthalpy of dissolution of kclo4(s) in kj/mol. assume the specific heat of the solution is equal to the specific heat of water.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1
You know the right answer?
In the laboratory a general chemistry student finds that when 2.84 g of kclo4(s) are dissolved in 10...
Questions in other subjects:


Spanish, 30.08.2021 18:40


English, 30.08.2021 18:40


Mathematics, 30.08.2021 18:40



Biology, 30.08.2021 18:40

History, 30.08.2021 18:40

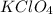 is 54.3 kJ/mole
is 54.3 kJ/mole![q=[q_1+q_2]](/tpl/images/0365/0174/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0365/0174/1d50b.png)
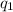 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter = heat absorbed by the water
= heat absorbed by the water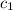 = specific heat of calorimeter =
= specific heat of calorimeter = 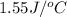
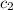 = specific heat of water =
= specific heat of water = 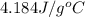
 = mass of water = 107.70 g
= mass of water = 107.70 g = change in temperature =
= change in temperature = 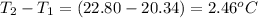
![q=[(1.55J/^oC\times 2.46^oC)+(107.70g\times 4.184J/g^oC\times 2.46^oC)]](/tpl/images/0365/0174/8f413.png)
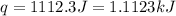
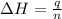
 = enthalpy change = ?
= enthalpy change = ?