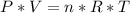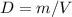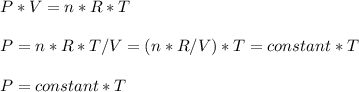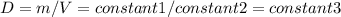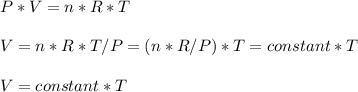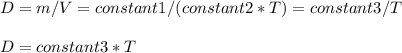Chemistry, 08.11.2019 03:31 kamkam5791
Consider two different containers, each filled with of . one of the containers is rigid and has constant volume. the other container is flexible (like a balloon) and is capable of changing its volume to keep the external pressure and internal pressure equal to each other. if you raise the temperature in both containers, what happens to the pressure and density of the gas inside each container? assume a constant external pressure.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Consider two different containers, each filled with of . one of the containers is rigid and has cons...
Questions in other subjects:


History, 22.04.2021 20:00



Mathematics, 22.04.2021 20:00



History, 22.04.2021 20:00