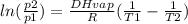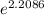Chemistry, 08.11.2019 01:31 jazz589729
Prior to the discovery that freon-12 (cf 2 cl 2 ) was harmful to the earth’s ozone layer, it was frequently used as the dispersing agent in spray cans for hair spray, etc. its enthalpy of vaporization at its normal boiling point of − 29.2°c is 20.25 kj mol − 1 . estimate the pressure that a can of hair spray using freon-12 had to withstand at 40°c, the temperature of a can that has been standing in sunlight. assume that ∆ vap h is a constant over the temperature range involved and equal to its value at − 29.2°c.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
Prior to the discovery that freon-12 (cf 2 cl 2 ) was harmful to the earth’s ozone layer, it was fre...
Questions in other subjects:

History, 24.05.2021 14:00

Mathematics, 24.05.2021 14:00



English, 24.05.2021 14:00

Chemistry, 24.05.2021 14:00

Mathematics, 24.05.2021 14:00

Mathematics, 24.05.2021 14:00