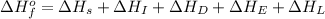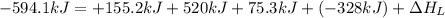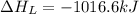Chemistry, 07.11.2019 22:31 kraigstlistt
The process of forming an ionic salt from its constituent metallic and nonmetallic elements is called the born-haber cycle, which is a series of thermochemical processes, each with a δh, that add up (think of hess’s law) to complete a 5 step process for the formation of the salt. given the following data, calculate the lattice energy per mole of lif(s) formed. li(s) → li(g) δh°

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1
You know the right answer?
The process of forming an ionic salt from its constituent metallic and nonmetallic elements is calle...
Questions in other subjects:



Mathematics, 06.05.2020 02:42





Mathematics, 06.05.2020 02:42

Mathematics, 06.05.2020 02:42

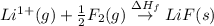
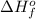 = enthalpy of formation of lithium fluoride = -594.1 kJ
= enthalpy of formation of lithium fluoride = -594.1 kJ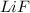 :
: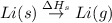
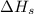 = sublimation energy of lithium = +155.2 kJ
= sublimation energy of lithium = +155.2 kJ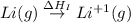
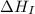 = ionization energy of lithium = +520 kJ
= ionization energy of lithium = +520 kJ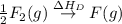
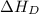 = dissociation energy of fluorine = +75.3 kJ
= dissociation energy of fluorine = +75.3 kJ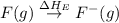
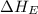 = electron affinity energy of fluorine = -328 kJ
= electron affinity energy of fluorine = -328 kJ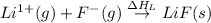
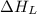 = lattice energy of lithium fluoride = ?
= lattice energy of lithium fluoride = ?