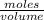Chemistry, 07.11.2019 22:31 nestergurl101
Then, calculate the molarity of a solution made by adding 5.3 g of cacl₂ to enough water to create a solution with a total volume of 2.50 l. enter only a numerical value in the correct number of significant figures. use the periodic table that is part of your exam to calculate molar mass. do not enter units in the answer box, but know that the symbol for the unit of this calculation is m.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 17:00, pinkypie123457
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 21.06.2019 23:00, slugmilk1090
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1
You know the right answer?
Then, calculate the molarity of a solution made by adding 5.3 g of cacl₂ to enough water to create a...
Questions in other subjects:


Mathematics, 05.04.2020 20:49


Mathematics, 05.04.2020 20:49


Mathematics, 05.04.2020 20:49


Mathematics, 05.04.2020 20:50

Computers and Technology, 05.04.2020 20:50