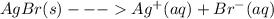Equilibrium shifts for slightly soluble compounds the reaction for the formation of a saturated solution of silver bromide (agbr) can be represented as agbr(s)? ag+(aq)+br? (aq)
consider that this reaction is an endothermic process and that the agbr solution is saturated.
classify the following changes based on whether they will favor the formation of reactant, whether they will favor the formation of products, or whether they will have no effect on the equilibrium.
1. adding hydrobromic acid (hbr)
2. lowering the concentration of bromide (br-) ions
3. heating the concentrations
4. adding solid silver bromide (agbr)
5. removing silver (ag+) ions

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, zamirareece17
1. list three scientific reasons cockroaches may fly.
Answers: 1

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Equilibrium shifts for slightly soluble compounds the reaction for the formation of a saturated solu...
Questions in other subjects:


Mathematics, 26.11.2019 03:31



Health, 26.11.2019 03:31

Social Studies, 26.11.2019 03:31