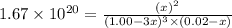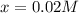Chemistry, 07.11.2019 06:31 winterblanco
0.0200 m fe3+ is initially mixed with 1.00 m oxalate ion, c2o42-, and they react according to the equation: fe3+(aq) + 3 c2o42-(aq) ⇄ [fe(c2o4)3]3-(aq) kc = 1.67 × 1020 what is the concentration of fe3+(aq) when equilibrium is reached? 1.67 × 1020 m 8.35 × 10-19 m 6.9a × 1021 m 1.44 × 10-22 m 0.980 am 0.940 m 0.0100 atm

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1
You know the right answer?
0.0200 m fe3+ is initially mixed with 1.00 m oxalate ion, c2o42-, and they react according to the eq...
Questions in other subjects:

History, 27.05.2021 16:40

Mathematics, 27.05.2021 16:40

Chemistry, 27.05.2021 16:40


Mathematics, 27.05.2021 16:40

Mathematics, 27.05.2021 16:40

English, 27.05.2021 16:40

Mathematics, 27.05.2021 16:40

Chemistry, 27.05.2021 16:40

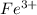 at equilibrium is 0 M.
at equilibrium is 0 M.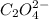 = 1.00 M
= 1.00 M![Fe^{3+}(aq)+3C_2O_4^{2-}(aq)\rightleftharpoons [Fe(C_2O_4)_3]^{3-}(aq)](/tpl/images/0363/4634/1f551.png)
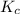 will be,
will be,![K_c=\frac{[[Fe(C_2O_4)_3]^{3-}]}{[C_2O_4^{2-}]^3[Fe^{3+}]}](/tpl/images/0363/4634/137fa.png)